FDA Issues Early Alert on Trividia Glucometer Issue Linked to 114 Injuries
•February 19, 2026
0
Companies Mentioned
Why It Matters
Delayed response to high‑glucose alerts can cause severe health outcomes, making the FDA’s warning a pivotal reminder of device‑related patient safety risks and regulatory oversight.
Key Takeaways
- •FDA alert links glucometer error code to 114 injuries.
- •Error occurs at glucose >600 mg/dL or strip malfunction.
- •Trividia updated instructions urging immediate medical care.
- •Devices stay in use; no mandatory recall.
- •Issue affects True Metrix meters worldwide and pharmacy brands.
Pulse Analysis
Glucometers are a cornerstone of diabetes management, providing real‑time data that guides insulin dosing and lifestyle decisions. When a device fails to flag dangerously high glucose levels—especially above 600 mg/dL—the clinical window for intervention narrows dramatically. The FDA’s early alert on Trividia’s True Metrix line underscores how even a seemingly minor error code can translate into life‑threatening delays, highlighting the critical intersection of device engineering, user interface design, and patient education in chronic disease care.
The core risk stems from patients interpreting the E‑5 error as a simple technical glitch rather than a physiological emergency. By updating the instructions to explicitly advise seeking medical attention when symptoms accompany the code, Trividia aims to mitigate the behavioral gap that contributed to the 114 reported injuries. This move also serves as a defensive strategy, reducing liability exposure while reinforcing the importance of clear, actionable labeling. Healthcare providers must now reinforce these guidance points during consultations, ensuring that users understand when an error warrants urgent clinical evaluation.
For manufacturers and pharmacy partners, the incident signals a broader imperative to embed safety nets within device software and documentation. Regulatory bodies are increasingly scrutinizing post‑market performance, and proactive communication—like Trividia’s instruction revision—can preserve market access across regions such as the U.S., Europe, and Australia. Retailers should train staff to convey these updates, while clinicians might consider supplemental monitoring tools for high‑risk patients. Ultimately, the episode illustrates how vigilant oversight and transparent user guidance can safeguard patient outcomes and sustain confidence in medical device ecosystems.
FDA issues early alert on Trividia glucometer issue linked to 114 injuries
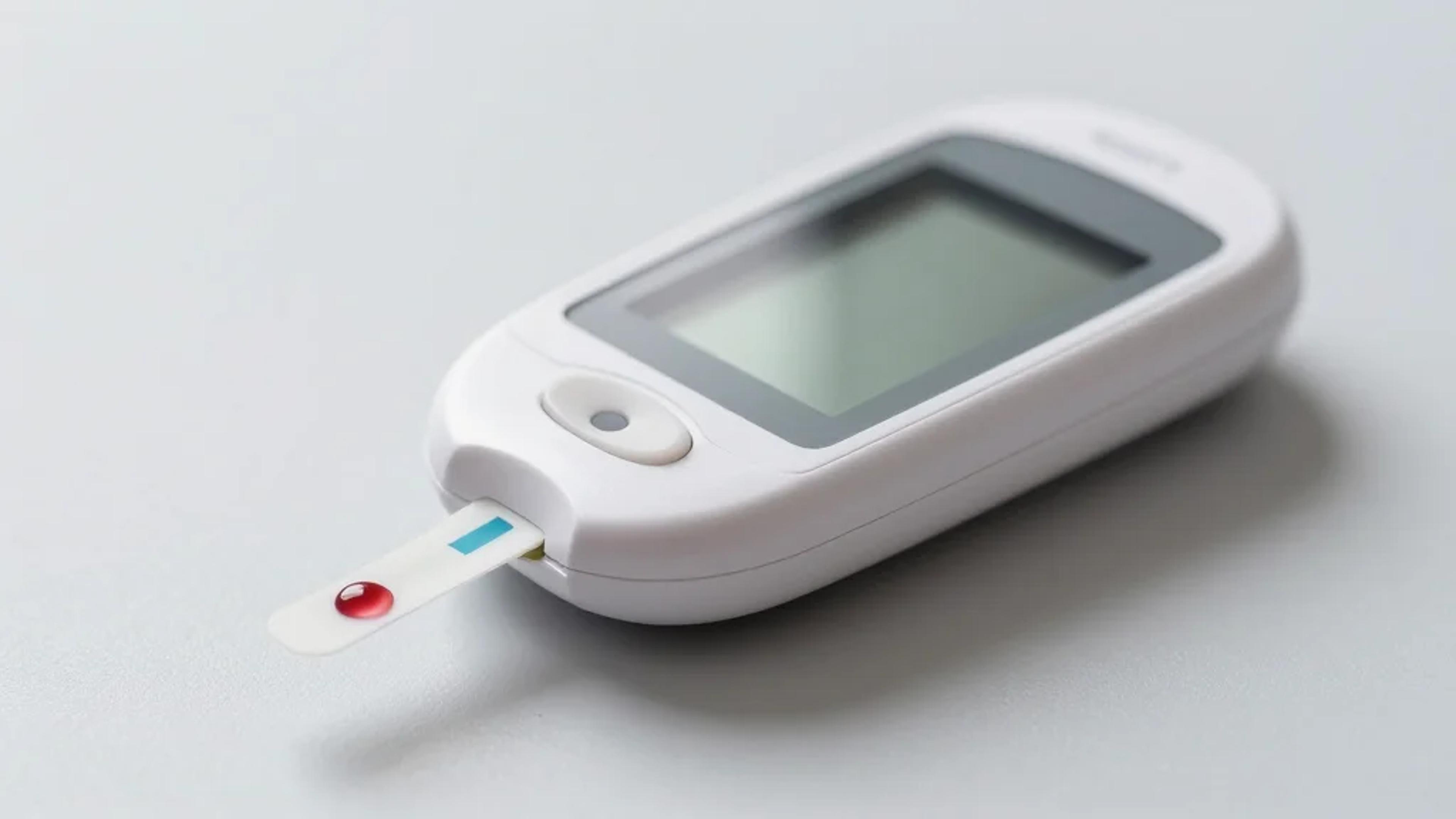
Users may receive an error code either due to a test strip error or a very high blood glucose event, Trividia Health said.
Trividia Health corrected the instructions for some of its blood glucose meters, including the True Metrix Air and True Metrix Self brands. Courtesy of Trividia Health
Dive Brief
-
The affected products may issue an error code in the case of a very high blood glucose result (higher than 600 mg/dL) or in the event of a test strip error, according to the FDA alert.
-
Earlier this month, Trividia issued a correction for four versions of its True Metrix blood glucose system. The company updated the devices’ instructions for use to clarify that patients should seek medical attention if they have symptoms of high glucose and receive an error code.
-
The Food and Drug Administration posted an early alert Tuesday for a problem with certain Trividia Health blood glucose monitors linked to 114 injuries and one death.
Dive Insight
The FDA uses early alerts to inform the public of potentially high‑risk device issues. Trividia updated the instructions for its blood glucose meters because they could lead to a delay in treatment if users don’t seek medical attention when they receive an E‑5 error code and have symptoms of high blood glucose, such as fatigue, thirst or blurry vision.
Delaying treatment could have serious health consequences for people with very high blood glucose levels, including dehydration, altered mental status or death, according to the early alert.
The correction applies to all True Metrix‑branded blood glucose meters distributed in the U.S., Mexico, the U.K., Australia and the Caribbean. It also affects co‑branded products sold in pharmacies including CVS, Kroger and Walgreens, according to Trividia’s letter to customers. The injuries and deaths were reported since August 2014, when Trividia first launched the devices. The injuries and death reports are as of Jan. 16, according to the FDA.
The True Metrix devices don’t need to be returned or replaced, and patients may continue to use them, according to the alert.
Trividia recalled some of its True Metrix monitors last year over concerns that some of the devices may have defective LCD displays.
0
Comments
Want to join the conversation?
Loading comments...