Medtronic Secures FDA Approval for Stealth AXiS Spine Robotics Platform
•February 16, 2026
0
Why It Matters
The clearance positions Medtronic to accelerate adoption of robot‑assisted spine procedures, potentially improving surgical precision while lowering operating costs and expanding its market share in a rapidly growing med‑tech segment.
Key Takeaways
- •FDA cleared Stealth AXiS integrates robotics, planning, navigation.
- •LiveAlign tracking reduces intra‑operative imaging needs.
- •Modular design supports future cranial and ENT extensions.
- •AiBLE ecosystem enables seamless data sharing across procedures.
- •Potential to lower costs and improve spine surgery outcomes.
Pulse Analysis
Spine surgery remains one of the most complex and resource‑intensive specialties, driving hospitals to seek technologies that enhance precision and efficiency. Over the past decade, robot‑assisted platforms have moved from niche tools to mainstream options, with market analysts projecting double‑digit growth through 2030. Medtronic’s entry with Stealth AXiS leverages its extensive device portfolio and aligns with the broader industry shift toward integrated, data‑driven operating rooms, positioning the company to capture a larger slice of the burgeoning robotics market.
At the heart of Stealth AXiS is LiveAlign segmental tracking, which continuously maps vertebral motion and patient alignment, allowing surgeons to adjust in real time without repeated fluoroscopic checks. This capability not only cuts radiation exposure but also shortens procedure times, translating into lower per‑case costs. The platform’s modular design means hospitals can start with core spine functionality and later add cranial or ENT modules as clinical needs evolve, reducing upfront capital expenditure while preserving upgrade pathways. Integration within Medtronic’s AiBLE ecosystem further streamlines workflow by linking pre‑operative planning, intra‑operative navigation, and post‑operative analytics on a unified data platform.
From a business perspective, FDA clearance unlocks immediate revenue opportunities in the U.S., the world’s largest spine‑surgery market. It also strengthens Medtronic’s competitive stance against rivals such as Intuitive Surgical and Zimmer Biomet, who are expanding their robotic offerings. The ability to bundle Stealth AXiS with existing Medtronic implants and software creates cross‑selling potential, while the prospect of future 510(k) extensions into cranial and ENT domains promises a broader addressable market. As hospitals prioritize value‑based care, technologies that improve outcomes and reduce costs—like Stealth AXiS—are likely to see accelerated adoption, reinforcing Medtronic’s growth trajectory in the med‑tech sector.
Medtronic secures FDA approval for Stealth AXiS spine robotics platform
February 2026
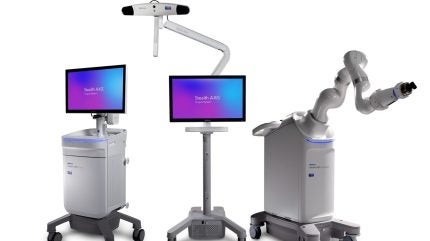
Medtronic has received the US Food and Drug Administration (FDA) clearance for the Stealth AXiS spine‑surgery robotics platform. The system is designed as an integrated solution that combines robotics, planning, and navigation in a single platform for spine procedures in the United States.
Medtronic aims to enable hospitals and ambulatory surgery centres to perform these procedures without relying on multiple separate technologies. The system’s architecture allows support for a variety of surgeon preferences and clinical complexities.
The company has built the platform to meet current needs while offering the possibility of future expansion to cranial and ear, nose, and throat (ENT) applications, subject to further 510(k) clearance. Its modular design lets institutions deploy only what they need now and scale capabilities as clinical requirements evolve.
A significant feature of the Stealth AXiS system is LiveAlign segmental tracking, which provides real‑time visualisation of anatomical movement, surgical adjustments, and patient alignment during spine surgery. This functionality reduces the need for repeated imaging and minimises reliance on manual workflow steps, supporting consistent execution of patient‑specific surgical plans.
The Stealth AXiS system forms a core part of Medtronic’s AiBLE smart ecosystem by integrating planning, navigation, and execution into a single workflow. This approach streamlines surgical processes and enables seamless information sharing before, during, and after surgery. The native connectivity within the AiBLE ecosystem is designed to link devices, software, and data to facilitate more meaningful insight exchange across the surgical continuum.
“Spine surgery is complex, and variability remains a real challenge.
The Stealth AXiS system is designed to make advanced technology more usable and clinically meaningful, helping surgeons deliver more predictable, personalised care while laying the foundation for continued innovation.”
— Michael Carter, Medtronic senior vice‑president and cranial and spinal technologies president
Earlier this month, Medtronic reported three milestones in the United States, expanding access to its MiniMed 780G insulin‑delivery system for individuals with type 1 diabetes (T1D) and insulin‑requiring type 2 diabetes (T2D).
0
Comments
Want to join the conversation?
Loading comments...