News•Feb 19, 2026
Cetirizine and Levocetirizine Withdrawal Pruritus: What Pharmacists Need to Know About the FDA’s New Safety Warning
The FDA will update labeling for cetirizine and levocetirizine to warn of a rare but serious withdrawal‑related pruritus that can emerge days after stopping long‑term use. More than 200 adverse‑event reports, primarily linked to cetirizine, describe severe itching requiring medical intervention. The reaction appears confined to these piperazine antihistamines and is not observed across the broader second‑generation class. Pharmacists are urged to counsel patients on symptom monitoring, consider tapering strategies, and report cases through MedWatch.
By Pharmacy Times

News•Feb 19, 2026
Health Systems Must Connect with Patients in More Meaningful Ways
Health systems are accelerating digital transformation post‑COVID, with HIMSS research showing that hospitals possessing advanced digital maturity are 3.25 times more likely to earn higher safety grades and experience lower infection and adverse event rates. The pandemic also shifted patients from...
By Healthcare IT News (HIMSS Media)
News•Feb 19, 2026
2024-2025 COVID-19 Vaccines Provided Moderate Protection Against JN.1 Variants
A recent JAMA Network Open case‑control study of 8,493 hospitalized adults shows that 2024‑2025 COVID‑19 vaccines provided moderate protection against the JN.1 lineage, with overall effectiveness of 40% against hospitalization and up to 52% after 90‑179 days. Updated Moderna and...
By AJMC (The American Journal of Managed Care)

News•Feb 19, 2026
Insulin Affordability: Policies and Pharmacist Roles in Diabetes Management
Insulin’s list price has surged more than 300% over the past two decades, driven by a rebate‑heavy gross‑to‑net bubble and limited biosimilar competition. Pharmacy‑benefit managers (PBMs) dominate 79% of the market, using high‑list‑price rebates to secure formulary placement, which inflates...
By Pharmacy Times

News•Feb 19, 2026
Navigating GLP-1 Costs With Eric Levin: Insurance, Cash Pay, and the Oral Wegovy Shift
Eric Levin, CEO of Scripta, explains that the newly launched oral Wegovy pill is typically cheaper on a cash‑pay basis—by a few hundred dollars per month—than injectable GLP‑1s, but insurance reimbursements are currently similar for both forms. Coverage depends heavily...
By AJMC (The American Journal of Managed Care)
News•Feb 19, 2026
LPM-5140276 Shows Enhanced Antitumor Efficacy in Combination with RMC-4550
Researchers reported that the novel KRAS G12D inhibitor LPM-5140276 markedly improves antitumor activity when paired with the SHP2 inhibitor RMC-4550. The combination demonstrated synergistic tumor regression in preclinical models of pancreatic and colorectal cancers harboring KRAS G12D mutations. Data suggest enhanced pathway...
By BioWorld (Citeline) – Featured Feeds

News•Feb 19, 2026
Nevada Debuts Public Option Amid Tumultuous Federal Changes to Health Care
Nevada launched its Battle Born public option health plans last fall, aiming to lower premiums and expand coverage. Early enrollment reached just over 10,000 members, far short of the 35,000 target set by state officials. The program must cut premiums...
By KFF Health News (formerly Kaiser Health News)

News•Feb 19, 2026
Bone Marrow Cell Atlas Created for Improved Leukemia Research
Researchers at the Princess Máxima Center have produced the first multimodal single‑cell atlas of healthy pediatric bone marrow, profiling nearly 91,000 cells from nine donors aged two to 32. The atlas reveals that children’s marrow differs markedly from adult marrow in...
By Medical Xpress
News•Feb 18, 2026
Researchers Develop RNA-Activated Implant to Stimulate Nerve Regrowth After Spinal Cord Injury
Researchers at RCSI have created a 3‑D biomaterial implant that releases PTEN‑targeting siRNA to injured spinal cord neurons, reactivating growth pathways. The scaffold replicates spinal cord mechanical properties and delivers RNA particles directly to the lesion site, silencing the PTEN...
By Medical Xpress

News•Feb 18, 2026
Top HHS Officials Tout TEFCA Data-Sharing Framework As Central To MAHA
Top officials at the Department of Health and Human Services highlighted the Trusted Exchange Framework and Common Agreement (TEFCA) as a cornerstone of the "Make America Healthy Again" (MAHA) initiative. TEFCA, mandated by the 2016 bipartisan law, seeks to create...
By Inside Health Policy

News•Feb 18, 2026
CBO: Part D Contributed To Higher Medicare Spending Than Expected
The Congressional Budget Office’s latest 10‑year budget outlook reveals that Medicare spending will be higher than previously projected, largely due to the Part D prescription‑drug benefit. CBO estimates the drug benefit adds several hundred billion dollars to federal outlays over the...
By Inside Health Policy

News•Feb 18, 2026
Cassidy, GOP Colleagues File Brief In LA Case On Comstock Act
Senate health committee chair Bill Cassidy and 59 Republican lawmakers filed an amicus brief supporting Louisiana’s lawsuit that the FDA violated the 1870 Comstock Act by permitting telehealth prescribing of mifepristone. The brief argues the agency overstepped its authority, seeking...
By Inside Health Policy
News•Feb 18, 2026
Oz: Codifying MFN Deals Could Avert ‘Draconian’ Pricing Measures
Pfizer CEO Albert Bourla met with CMS Administrator Mehmet Oz to negotiate a "most‑favored‑nation" (MFN) drug pricing agreement, though the specific terms were not disclosed. Oz indicated that the Trump administration plans to codify such voluntary MFN deals into law...
By Inside Health Policy

News•Feb 18, 2026
Oz: Codifying MFN Deals Could Avert ‘Draconian’ Pricing Measures
Pfizer CEO Albert Bourla disclosed a direct collaboration with CMS Administrator Mehmet Oz on a "most favored nation" (MFN) drug pricing arrangement, though the specific terms remain undisclosed. Oz indicated that the Trump administration intends to codify such voluntary MFN...
By Inside Health Policy
News•Feb 18, 2026
Pediatric Myopia Drug Is Latest In String Of Unexpected FDA Rejections
The FDA has rejected a pediatric myopia drug, citing a lack of substantial evidence for its efficacy. Pediatric ophthalmologists argue the drug, already used in compounded form, effectively slows myopia progression in children. The decision follows a series of recent,...
By Inside Health Policy

News•Feb 18, 2026
Cutting-Edge Technologies Are Poised to Transform Orthopedics
Orthopedic surgery is rapidly adopting AI, robotic assistance, VR/AR and wearable digital tools, shifting from experimental concepts to routine practice. AI now supports imaging interpretation, predictive risk modeling, personalized surgical planning, training simulations and remote postoperative monitoring. Robotic platforms improve...
By Healio – All News

News•Feb 18, 2026
FDA Backtracks On Moderna mRNA Flu Vaccine Refusal, Sets Aug. 5 Review Deadline
The U.S. Food and Drug Administration has reversed its earlier decision to decline a review of Moderna's mRNA influenza vaccine candidate. Following a Type A meeting with the company, the agency set an August 5, 2026 deadline for completing its evaluation. The...
By Inside Health Policy

News•Feb 18, 2026
N.I.H. Director Will Temporarily Run C.D.C. in Leadership Shake-Up
Dr. Jay Bhattacharya, the current NIH director, has been named acting director of the CDC, a role he will hold while continuing to lead the National Institutes of Health. He replaces Jim O’Neill, who is slated for a nomination to...
By New York Times – Health
News•Feb 18, 2026
Identy Joins Africa’s Push for Digital Identity in Humanitarian Healthcare
Identy is partnering with the volunteer NGO HumanCoop to roll out offline facial‑recognition tools for undocumented patients in northern Mauritania, creating portable digital medical IDs that work without internet. The initiative will initially cover the Bir Mogrein community of over 2,500...
By Biometric Update

News•Feb 18, 2026
AI in Healthcare Starts Long Before the Exam Room
AI-driven clinical tools are rapidly improving diagnosis and treatment, but they leave patients exposed to hidden financial risks. A case of an autonomous AI ER physician resulted in a $37,000 out‑of‑network bill, highlighting systemic navigation failures. While clinical AI promises...
By Employee Benefit News

News•Feb 18, 2026
The Murky Waters of Healthcare Cost Transparency: What Plan Sponsors Can Control
The 2021 Consolidated Appropriations Act introduced mandatory broker compensation disclosures and broader pricing data for health plans, pushing plan sponsors to adopt fiduciary rigor similar to retirement plans. Yet, translating transparency into cost control remains difficult as procedure prices can...
By Human Resource Executive

News•Feb 18, 2026
Broker’s Call: Ipca Laboratories (Buy)
Ipca Laboratories posted a robust 19% year‑on‑year EBITDA increase to ₹530 crore, surpassing broker estimates, while revenue rose 6.6% to ₹2,400 crore. Domestic formulation sales grew 12% and export formulation climbed 17%, offset by flat API sales overall but a 26% rebound...
By The Hindu BusinessLine – Markets
News•Feb 18, 2026
Novartis' Oral BTK Drug Moves the Needle in CINDU
Novartis announced that its oral BTK inhibitor Rhapsido (remibrutinib) achieved significantly higher complete response rates than placebo in the phase 3 RemIND trial for the three most common forms of chronic inducible urticaria (CINDU). The drug, already approved for chronic spontaneous...
By pharmaphorum

News•Feb 18, 2026
NICE Backs First Disease-Modifying Drug for ARG1 Deficiency
Immedica’s pegzilarginase (Loargys), the first enzyme replacement therapy for arginase‑1 (ARG1) deficiency, has received NICE endorsement for NHS use in the UK. The weekly IV or subcutaneous treatment cuts blood arginine levels by roughly 80% and is recommended for patients...
By pharmaphorum

News•Feb 18, 2026
£20M Fund Backs UK Addiction Treatment Tech
The UK government, via Innovate UK, has launched a £20 million funding programme to accelerate development of medicines, medical devices, wearables, virtual‑reality therapies and AI‑enabled tools for drug and alcohol addiction. Grants of up to £10 million for late‑stage projects and up to...
By Startups Magazine

News•Feb 18, 2026
Six Biotechs to Know in Barcelona
Barcelona is emerging as a premier European life‑sciences hub, contributing 7.6 % of Catalonia’s GDP and ranking sixth in scientific output. Six local biotech firms—Accure Therapeutics, Oryzon Genomics, SpliceBio, Peptomyc, Ona Therapeutics and Integra Therapeutics—are advancing diverse modalities from small‑molecule neuroprotectors...
By Labiotech.eu

News•Feb 18, 2026
Extens-Backed Orthalis Picks up Dentalsoft
Orthalis, backed by private equity firm Extens, announced the acquisition of Dentalsoft, a specialist orthodontic software provider. The purchase creates a new entity, SignalSoft Développement, focused on delivering software services to orthodontic professionals. By integrating Dentalsoft’s tools, Orthalis expands its...
By PE Hub
News•Feb 18, 2026
AI and OCT Integration Highlights Promising Advances in Detecting Lipid-Rich Coronary Artery Plaques
Researchers at KAIST unveiled an AI‑driven method that extracts wavelength‑dependent information from standard optical coherence tomography (OCT) to automatically detect lipid‑rich coronary plaques. The weakly supervised deep‑learning model learns from frame‑level labels, eliminating the need for pixel‑wise annotations and works...
By Bioengineer.org

News•Feb 18, 2026
AI-Powered Liquid Biopsy Advances Pediatric Brain Tumor Classification
Researchers at St. Jude Children’s Research Hospital introduced M‑PACT, an AI‑driven liquid‑biopsy platform that classifies pediatric brain tumors from cerebrospinal fluid with 92% accuracy. The deep neural network was trained on over 5,000 DNA methylation profiles covering about 100 tumor...
By Bioengineer.org
News•Feb 18, 2026
Kipu Health Launches Elevate 2026
Kipu Health announced Elevate 2026, a three‑day customer conference in Carlsbad, California, showcasing its AI‑native Kipu Helix Intelligent Operating System for behavioral health. The event gathers executives, operators and administrators to explore AI‑driven strategies, hands‑on programming and peer insights. Kipu...
By AI-TechPark

News•Feb 18, 2026
Ongoing Regulatory Uncertainty Means Innovation Is Now Higher Risk
Regulatory uncertainty is rising as the FDA shifts leadership, adopts a single‑trial approval pathway, and tightens its benefit‑risk framework. While the one‑trial standard promises faster, cheaper market entry, recent surprise complete response letters (CRLs) show that even successful Phase 3 results...
By BioSpace

News•Feb 18, 2026
Ionis Paves Its Own Path as Initial Tryngolza Launch Defies Expectations
Ionis Therapeutics announced that its GSK‑partnered antisense drug bepirovirsen achieved a statistically significant functional cure rate of roughly 15‑20% in a Phase 3 trial, far surpassing the 1‑3% rate of existing hepatitis B therapies. The company also reported a strong first‑year launch...
By BioSpace

News•Feb 18, 2026
Proliferating Patents, Lawsuits Stave Off Pharmas’ Generic Competitors
A new analysis in Health Affairs Scholar reveals that brand‑name drugmakers are exploiting the Hatch‑Waxman framework through serial patent litigation and continuation patents to extend market exclusivity. By filing multiple overlapping patents, companies can repeatedly sue generic challengers, triggering 30‑month...
By BioSpace
News•Feb 17, 2026
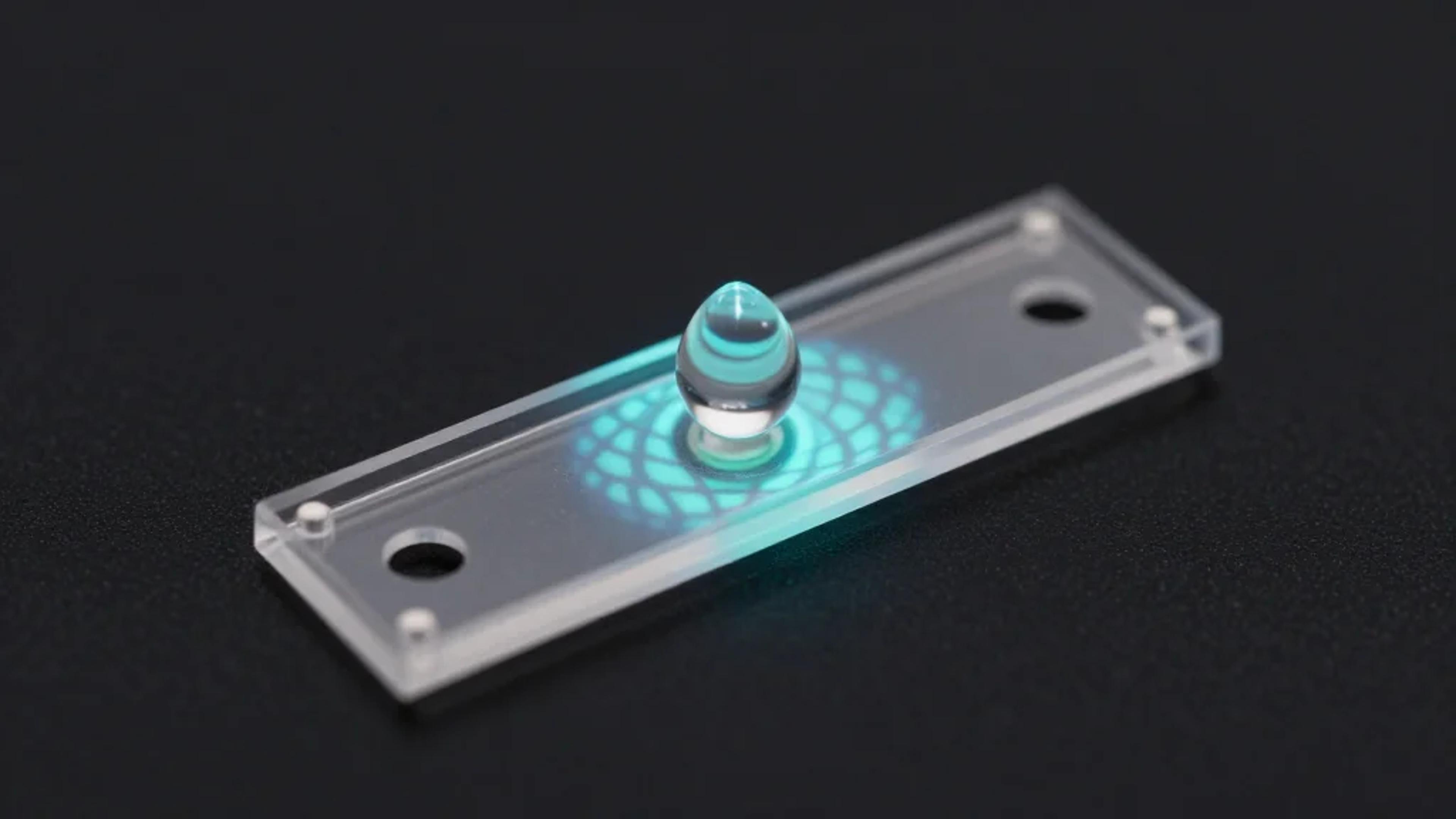
Projecting Light to Dispense Liquids: A New Route to Ultra-Precise Microdroplets
Researchers at Southern University of Science and Technology have unveiled an optoelectrowetting platform that uses programmable light patterns to dispense nanoliter droplets with unprecedented precision. By projecting dynamic illumination onto a microfluidic chip, the system creates virtual electrodes that guide...
By Nanotech Now

News•Feb 17, 2026
From Sensors to Smart Systems: The Rise of AI-Driven Photonic Noses
The 2025 review in *Microsystems & Nanoengineering* details how photonic noses combine optical sensing with AI to create highly selective, drift‑free chemical detectors. By leveraging colorimetric, refractive‑index, absorption and spectroscopy techniques, these devices generate rich spectral fingerprints that machine‑learning models...
By Nanotech Now

News•Feb 17, 2026
HIMSS26: Former AMA President Dr. Jesse Ehrenfeld Gives Keynote to Kick Off Physicians’ Forum
Dr. Jesse M. Ehrenfeld, former AMA president and global CMO of Aidoc, opened HIMSS26’s Physicians’ Forum with a keynote titled “From Hype to Healing: Real‑World AI Integration in Clinical Practice.” He warned that clinicians are overwhelmed and that AI must...
By Healthcare Finance News (HIMSS Media)
News•Feb 17, 2026
Medtronic Completes First Hugo Case in the US
Medtronic performed the first U.S. Hugo robotic surgery—a prostatectomy at the Cleveland Clinic—shortly after receiving FDA clearance. The system, already CE‑marked in Europe, is being installed at Duke University Hospital and Atrium Health, marking its initial U.S. footprint. CEO Geoff...
By MedTech Dive

News•Feb 17, 2026
Telehealth for Primary Care Levels Off: Epic
Epic’s analysis of 411 million primary‑care visits shows telehealth usage has plateaued at roughly 6‑7 % of appointments since mid‑2023, following the pandemic‑driven surge. The share remains highest in metropolitan areas, with lower adoption in micropolitan, rural and small‑town settings. Adults aged...
By Becker’s Hospital Review

News•Feb 17, 2026
Fitch Downgrades Missouri Hospital to ‘D’ Rating
Fitch downgraded John Fitzgibbon Memorial Hospital in Marshall, Missouri to a “D” rating from “C” and withdrew its issuer and bond ratings after the hospital defaulted on required debt payments. The default concerns principal and interest on 2010 bonds, and...
By Becker’s Hospital Review

News•Feb 17, 2026
Marshfield Clinic to Open 13th Hospital March 1
Marshfield Clinic will open its 13th hospital on March 1 in Wisconsin Rapids, adding a full‑service acute‑care facility to the existing Marshfield Clinic Wisconsin Rapids Center. The new campus features inpatient beds, an emergency department, advanced imaging such as CT...
By Becker’s Hospital Review

News•Feb 17, 2026
Cleveland Clinic 1st to Use New Robot for Prostate Surgery
Cleveland Clinic became the first U.S. health system to perform a robotic‑assisted prostatectomy using the newly FDA‑cleared Hugo RAS system. The robot, approved in December, features modular arms and an open‑console that surgeons control with foot pedals and hand inputs....
By Becker’s Hospital Review

News•Feb 17, 2026
Fraud In The Care Economy Morphs From Solo Scams To Global Syndicates
The care economy’s expansion has attracted a sophisticated, global fraud network that stole nearly $4.9 billion from older Americans in 2024, a three‑fold rise over five years. Scammers now run multi‑stage attacks that blend tech‑support ruses, fake bank officials, and crypto...
By Forbes – Healthcare

News•Feb 17, 2026
Building on a Foundation of Culture and Trust: How Jefferson Health Reopened a Hospital Just 9 Days After a Fire
On Feb. 4, a fire engulfed the Orthopedic Institute building at Jefferson Health’s Lehigh Valley Hospital‑Dickson City, prompting the evacuation of more than 70 patients. Thanks to extensive disaster training and coordinated effort among staff, first responders, and local partners, no...
By Becker’s Hospital Review

News•Feb 17, 2026
Jefferson Posts $201M Operating Loss in H1
Thomas Jefferson University, owner of Jefferson Health, posted a $201 million operating loss for the first half of fiscal 2026, reflecting a -2.3% operating margin. The loss includes $64.7 million in restructuring expenses tied to a planned layoff of roughly 650 employees....
By Becker’s Hospital Review

News•Feb 17, 2026
Cosmetic Manufacturers Pty Ltd. - 719225 - 02/06/2026
The U.S. Food and Drug Administration issued Warning Letter 320‑26‑43 to Cosmetic Manufacturers Pty Ltd., citing extensive Current Good Manufacturing Practice (CGMP) violations across laboratory testing, process control, equipment qualification, and written procedures. The agency placed the firm on Import...
By FDA – U.S. Food & Drug Administration (RSS Feeds)

News•Feb 17, 2026
Competitive Generic Therapy Approvals
The FDA’s Office of Generic Drugs has launched a public, bi‑weekly list of all approved abbreviated new drug applications (ANDAs) that carry a Competitive Generic Therapy (CGT) designation. The list details each product’s reference listed drug, NDA number, applicant, active...
By FDA – U.S. Food & Drug Administration (RSS Feeds)
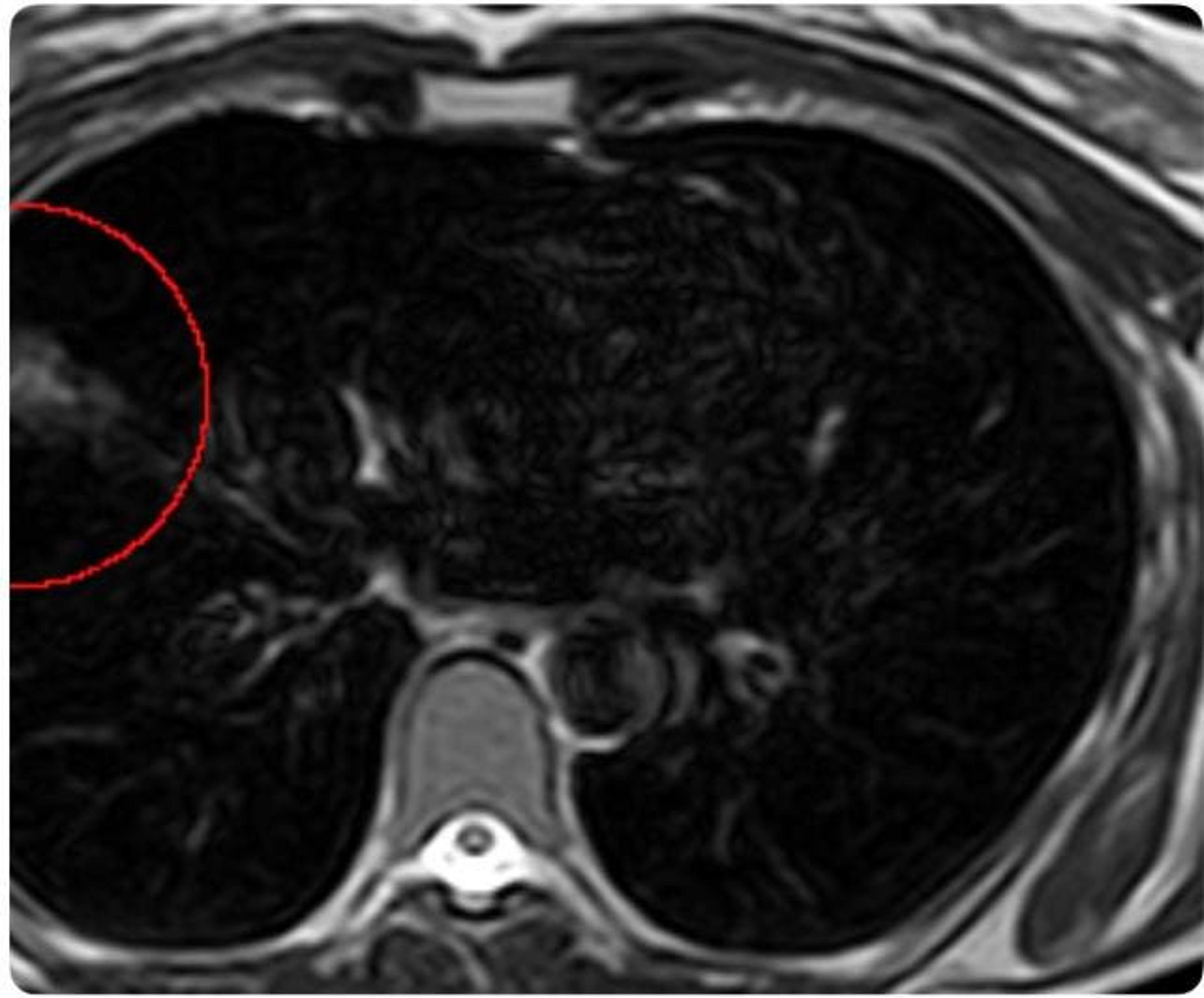
News•Feb 17, 2026
Lung Cancer Rising Among Never-Smokers. Screening For This Group Lags.
Never‑smokers now account for 10‑20% of U.S. lung‑cancer cases, a share that is climbing despite overall declines. Current USPSTF guidelines limit low‑dose CT screening to heavy‑smoking adults, leaving most never‑smokers unscreened. Shira Boehler’s incidental finding on a whole‑body MRI prompted...
By Forbes – Healthcare

News•Feb 17, 2026
Autism In Women May Be As Common As In Men, Study Finds
A large Swedish cohort study of 2.7 million children tracked over 35 years shows that while boys are diagnosed more often in childhood, the male‑to‑female autism ratio narrows to about 1.2 by age 20 and may reach parity in adulthood. The...
By Forbes – Healthcare

News•Feb 17, 2026
Selecting the Optimal Cell Therapy Manufacturing Platform
Developers of cell and gene therapies must choose between fully integrated turnkey platforms and modular, multi‑device systems. Integrated solutions deliver rapid time‑to‑value and simplified workflows, ideal for low‑volume autologous products, but they restrict reagent flexibility and scale. Modular platforms provide...
By BioPharma Dive

News•Feb 17, 2026
How to Evaluate Prospective Diagnostic Laboratory Partners for Your Patient Support Program
The pharmaceutical industry now invests over $5 billion annually in patient support programs (PSPs), which boost adherence by roughly 30 % and lower overall healthcare costs. Diagnostic laboratory testing is a cornerstone of these programs, delivering timely data that guides dosing, monitors...
By BioPharma Dive