Antigen Orientation Boosts HPV Cancer SNA Vaccine, Slows Tumors in Models
•February 13, 2026
0
Why It Matters
The findings prove that nanostructural design, not just composition, can dramatically boost therapeutic vaccine efficacy, opening a pathway to more effective treatments for HPV‑driven cancers and potentially other tumors.
Key Takeaways
- •N-terminal antigen display dramatically increases T‑cell response
- •N‑HSNA cut tumor burden threefold in mouse model
- •Structural orientation outperforms traditional peptide‑adjuvant mixes
- •Design shows promise for therapeutic HPV cancer vaccines
- •Blueprint may revive failed vaccine candidates via nanostructure tuning
Pulse Analysis
The emerging discipline of structural nanomedicine is reshaping how researchers think about vaccine design. By arranging a single peptide on a spherical nucleic acid scaffold with atomic‑scale precision, scientists can modulate how immune cells recognize and process the antigen. The Northwestern study highlights that a modest shift—exposing the HPV16 E7 epitope at the nanoparticle’s exterior—produces a cascade of immunological benefits, from heightened dendritic cell activation to robust CD8⁺ T‑cell cytotoxicity. This contrasts sharply with the conventional "blender" approach where components are simply mixed together.
In preclinical models, the N‑terminally displayed SNA (N‑HSNA) outperformed both buried‑peptide and C‑terminal configurations. Mice bearing HPV‑positive head and neck tumors experienced a threefold reduction in tumor volume and a statistically significant survival advantage. Human‑derived tumor spheroids mirrored these results, showing a 2.5‑fold increase in cancer cell killing. These outcomes suggest that precise antigen orientation can bridge the efficacy gap between prophylactic HPV vaccines, which prevent infection, and therapeutic vaccines needed for established malignancies.
Looking ahead, the study provides a template for rational vaccine engineering across oncology. Integrating computational modeling and machine‑learning algorithms could accelerate the identification of optimal nanostructures for diverse tumor antigens. For biotech firms, this approach offers a route to revitalize previously failed vaccine candidates by revisiting their architectural layout rather than overhauling their molecular composition. As regulatory pathways adapt to nanomedicine, investors and developers should monitor structural design as a differentiator in the competitive therapeutic vaccine landscape.
Antigen Orientation Boosts HPV Cancer SNA Vaccine, Slows Tumors in Models
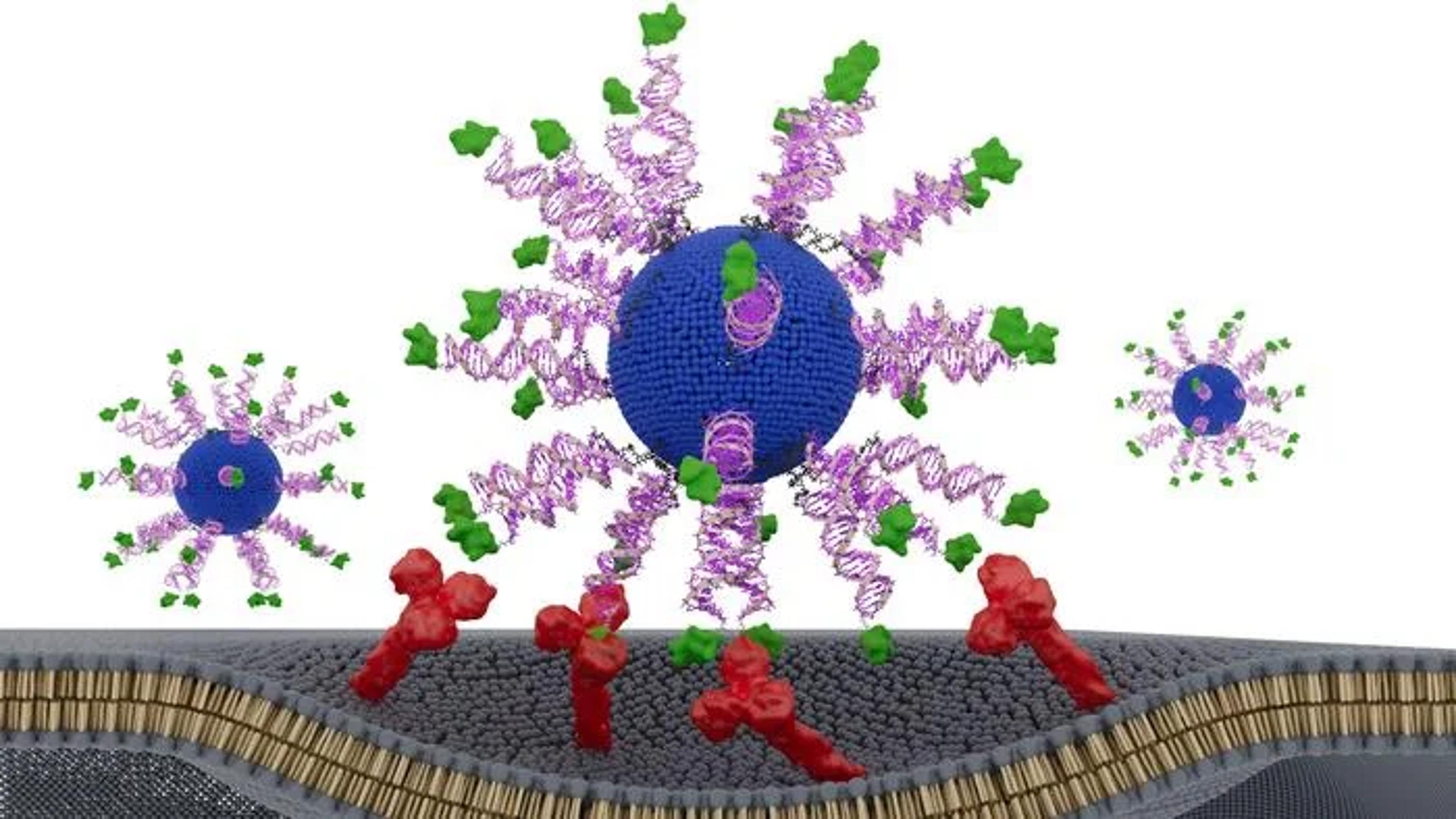
Biologists often say that structure determines function. In a new study, Northwestern University researchers show that this principle applies not only to proteins but may also to cancer vaccines—where the nanoscale arrangement of a single peptide can dramatically alter therapeutic potency. By re‑engineering the orientation of an HPV‑derived antigen on a spherical nucleic acid (SNA) platform, the team created a vaccine that slowed tumor growth and extended survival in preclinical models of HPV‑driven cancer.
The work, published in Science Advances and titled “E711‑19 Placement and Orientation Dictate CD8⁺ T Cell Response in Structurally Defined Spherical Nucleic Acid Vaccines,” explores how subtle architectural changes can reshape immune activation. HPV‑positive head and neck cancers, which are rising in incidence, often present at advanced stages and are treated with toxic regimens, according to the authors. While prophylactic HPV vaccines prevent infection, they do not help patients with established tumors—leaving a need for therapeutic strategies that can safely drive strong cytotoxic T‑cell responses.
To address this, the researchers designed three SNA‑based vaccines containing identical components: a lipid core, CpG adjuvant, and a short HPV16 E711‑19 peptide. The only difference was how the antigen was displayed. One formulation buried the peptide inside the nanoparticle, while two others attached it to the surface through either the N‑terminus or C‑terminus—a small structural shift with potentially large immunological consequences.
The differences were striking. All three SNAs enhanced dendritic cell activation and CD8⁺ T‑cell cytotoxicity compared to a simple peptide‑adjuvant mixture, but the N‑terminally displayed version, dubbed N‑HSNA, consistently outperformed the others. It induced roughly eight‑fold higher interferon‑γ secretion and about 2.5‑fold greater cytotoxicity in primary human cells, the authors wrote.
In tumor‑bearing AAD mice, the N‑HSNA formulation cut tumor burden by more than threefold and extended survival, while also driving a noticeable expansion of CD8⁺ T cells. The same design performed strongly in patient‑derived HPV‑positive head and neck cancer spheroids, where it produced roughly a 2.5‑fold increase in tumor‑cell killing—a sign that the structural tuning may translate across model systems.
The findings highlight the emerging field of “structural nanomedicine,” which emphasizes the deliberate arrangement of vaccine components rather than simply mixing them together—what lead author Chad A. Mirkin, PhD, calls the “blender approach.”
“There are thousands of variables in the large, complex medicines that define vaccines,” said Mirkin, the George B. Rathmann Professor at Northwestern. “The promise of structural nanomedicine is being able to identify the configurations that lead to the greatest efficacy and least toxicity. In other words, we can build better medicines from the bottom up.”
By demonstrating that antigen placement and orientation can dictate immune potency, the study provides a blueprint for revisiting past cancer vaccines that failed not because of their ingredients, but perhaps because of their architecture. The authors suggest that rational design—potentially aided by machine learning—could accelerate the development of more effective therapeutic vaccines for HPV‑driven tumors and beyond.
The post Antigen Orientation Boosts HPV Cancer SNA Vaccine, Slows Tumors in Models appeared first on GEN - Genetic Engineering and Biotechnology News.
0
Comments
Want to join the conversation?
Loading comments...