Terray’s AI Model Accelerates Drug Potency Prediction, Pose Not Needed
•February 12, 2026
0
Why It Matters
Accelerating potency prediction shortens drug‑discovery cycles, lowering costs and expanding access to novel therapeutics. The breakthrough positions Terray as a competitive player in AI‑driven pharma pipelines.
Key Takeaways
- •TerraBind improves potency prediction accuracy by ~20%
- •Model screens 26× more chemical space with same resources
- •Bypasses pose generation, unlike AlphaFold and Boltz models
- •Terray's dataset holds 14 billion binding measurements
- •First clinical candidate aimed for 2027
Pulse Analysis
AI‑driven drug discovery has long wrestled with the trade‑off between structural fidelity and throughput. Traditional models such as AlphaFold and the Boltz series compute full atom‑level poses before estimating binding affinity, a process that consumes significant compute cycles. TerraBind sidesteps this bottleneck by directly forecasting potency, effectively decoupling predictive power from pose generation. This architectural shift not only trims computational overhead but also opens the door to evaluating millions of candidates that were previously out of reach.
The performance gains reported by Terray are striking: a 20% lift in prediction accuracy coupled with a 26‑fold increase in screening efficiency. Leveraging a proprietary ultra‑miniaturized chip, the company amasses a 14‑billion‑point binding affinity dataset, feeding the COATI reasoning engine that navigates unseen chemical space. This data‑rich environment fuels the Experimentation Meets Machine Intelligence (EMMI) platform, enabling rapid iteration from hit identification to lead optimization. As a result, Terray projects a three‑to‑four‑fold acceleration in overall pipeline speed, potentially halving the time required to bring a small‑molecule drug from concept to clinic.
From a business perspective, TerraBind strengthens Terray’s strategic partnerships with industry leaders like Bristol Myers Squibb, Calico, and Gilead. The recent BMS milestone underscores confidence in the platform’s ability to tackle “novel and difficult to drug” targets. With a brain‑penetrant multiple‑sclerosis inhibitor already in the pipeline and a target clinical entry in 2027, Terray is poised to translate its AI advantage into tangible market value. Investors and competitors alike will watch how this efficiency‑driven model reshapes the economics of early‑stage drug development.
Terray’s AI Model Accelerates Drug Potency Prediction, Pose Not Needed
Jacob Berlin, PhD, CEO of Terray Therapeutics, explains that the critical element to “get right” for a preclinical drug discovery campaign is identifying molecules that rank best among the candidate set.
The AI drug discovery company is building an end-to-end pipeline to bring novel small molecule drugs from unseen chemical space to the clinic. Designing these therapeutics from scratch, without patent information or public datasets, will require evaluating thousands to millions of molecules across the discovery process, from hit identification to lead optimization.
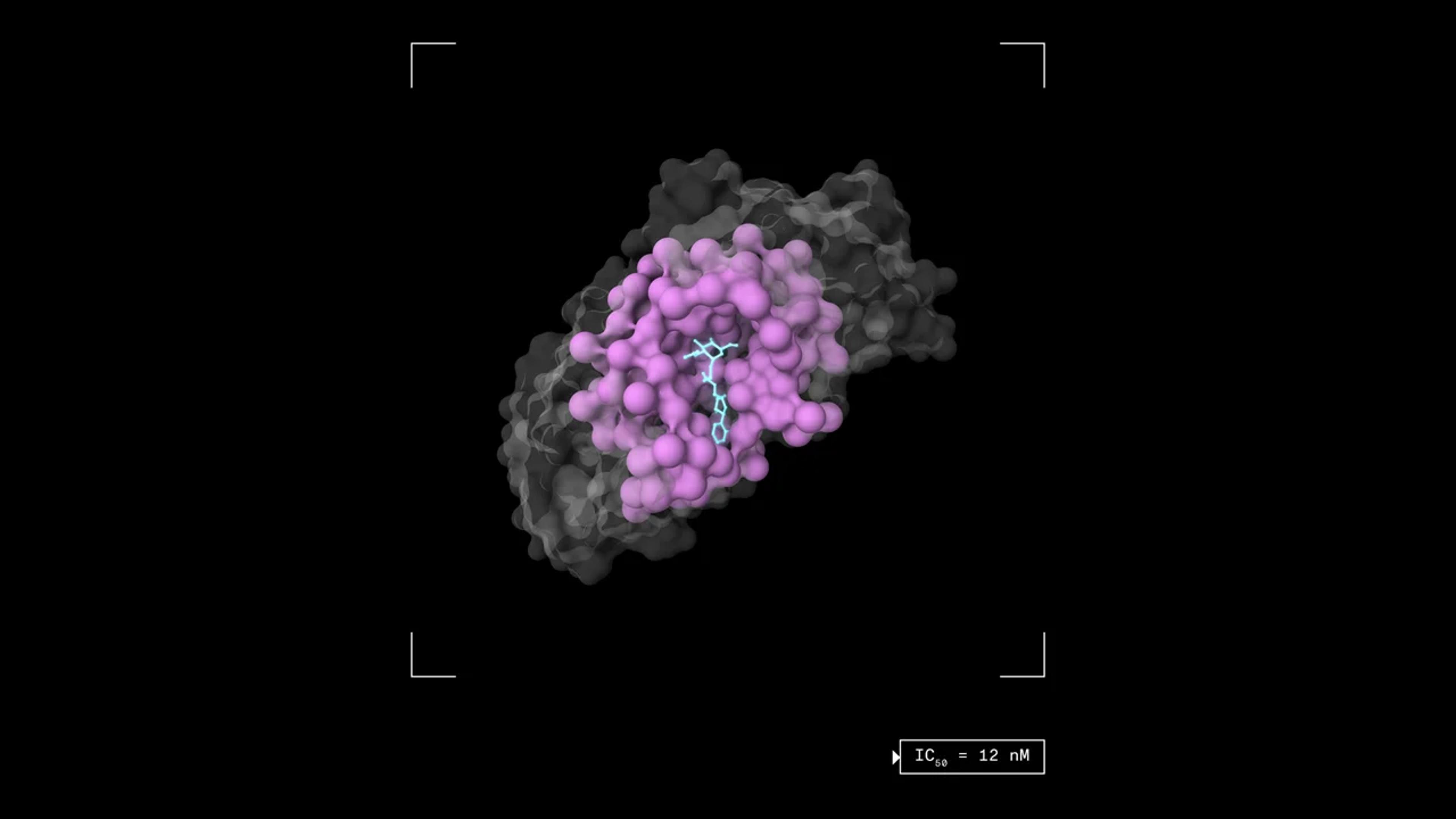
To support this goal, Terray has unveiled TerraBind, a small molecule potency prediction model that demonstrates an approximately 20% increase in accuracy and 26 times more efficiency gain when compared against Boltz-2, the widely adopted open-source binding affinity model developed by MIT researchers, who recently turned their models into a business.
Correspondingly, TerraBind permits screening 26 times more chemical space using the same resources. The model’s efficiency advantages stack up across the development pipeline, from expanding the therapeutic search space to achieving higher quality candidate selection for synthesis. The work is posted as a preprint on arXiv that has not yet been peer reviewed.
Unlike co-folding models, such as Nobel Prize-winning AlphaFold and the Boltz series of models, which use a computationally intensive all-atom approach to determine the structure of a molecule when bound to the target, TerraBind’s architecture bypasses this step to predict drug potency directly.
Berlin says there is a disconnect between the pictorial representation of a molecule and therapeutic function. This “snapshot in time” on its own does not provide information on binding affinity nor duration, key metrics for therapeutic effect.
“In the practicality of drug development, potency is more important. How much better will a change make this molecule?” Berlin posed in an interview with GEN Edge.
Based in Southern California’s Monrovia, Terray was founded in 2018 and has since solidified collaborations with Bristol Myers Squibb, Calico Life Sciences, Gilead and Odyssey to develop small molecule drugs spanning broad indications, including age-related diseases and transcription factor therapies.
In December, Terray achieved its first BMS milestone. While therapeutic details of the milestone have not been disclosed, Berlin describes the target as “novel and difficult to drug,” and representative of the company’s Experimentation Meets Machine Intelligence (EMMI) platform. Terray’s lead asset, a brain-penetrant inhibitor for multiple sclerosis, is also a product of EMMI.
Slowest we’ll ever go
EMMI is powered by data generated by Terray’s proprietary ultraminiaturized chip technology which produces one billion small molecule binding affinity measurements per quarter on average. The company describes its full 14 billion measurement dataset, as “the largest global database of binding data.”
Terray’s AI stack is grounded in a reasoning model, named COATI, which traverses unseen chemical space to achieve fine-grained, precision in structure with desired properties in drug discovery. Millions of candidate designs are computationally generated, providing an “AI abundance” for TerraBind to make potency predictions to inform molecule selection.
Berlin notes that Terray’s current platform is on track to double the speed by which small molecule drugs are developed from scratch. He says the current pace is the “slowest that Terray will ever go,” predicting pipeline efficiency to increase three to four times the speed now that EMMI is supported by 14 billion data points and a full AI stack.
Terray also expects to have its first candidate in the clinic in 2027.
The post Terray’s AI Model Accelerates Drug Potency Prediction, Pose Not Needed appeared first on GEN - Genetic Engineering and Biotechnology News.
0
Comments
Want to join the conversation?
Loading comments...