Airglove Medical Announces Major Breakthrough in Difficult Venous Access
•February 17, 2026
0
Why It Matters
Reducing first‑attempt failures cuts patient anxiety, shortens procedure times, and eases staff workload, delivering measurable efficiency gains for hospitals.
Key Takeaways
- •Airglove v2 uses warm air to dilate veins.
- •87.5% first‑time cannulation achieved in oncology trial.
- •UK sees 32 million cannulations annually, 30% fail first attempt.
- •System reduces procedure delays and staff workload.
- •Patent‑protected technology could reshape IV access market.
Pulse Analysis
Difficult venous access remains a persistent challenge in modern healthcare, especially in oncology where fragile veins and treatment urgency converge. In the UK alone, 32 million cannulations occur each year, yet nearly one‑third fail on the first attempt, leading to patient discomfort, increased infection risk, and costly procedural delays. Innovations that address the physiological barriers to successful cannulation are therefore high‑value assets for hospitals seeking to improve outcomes and operational efficiency.
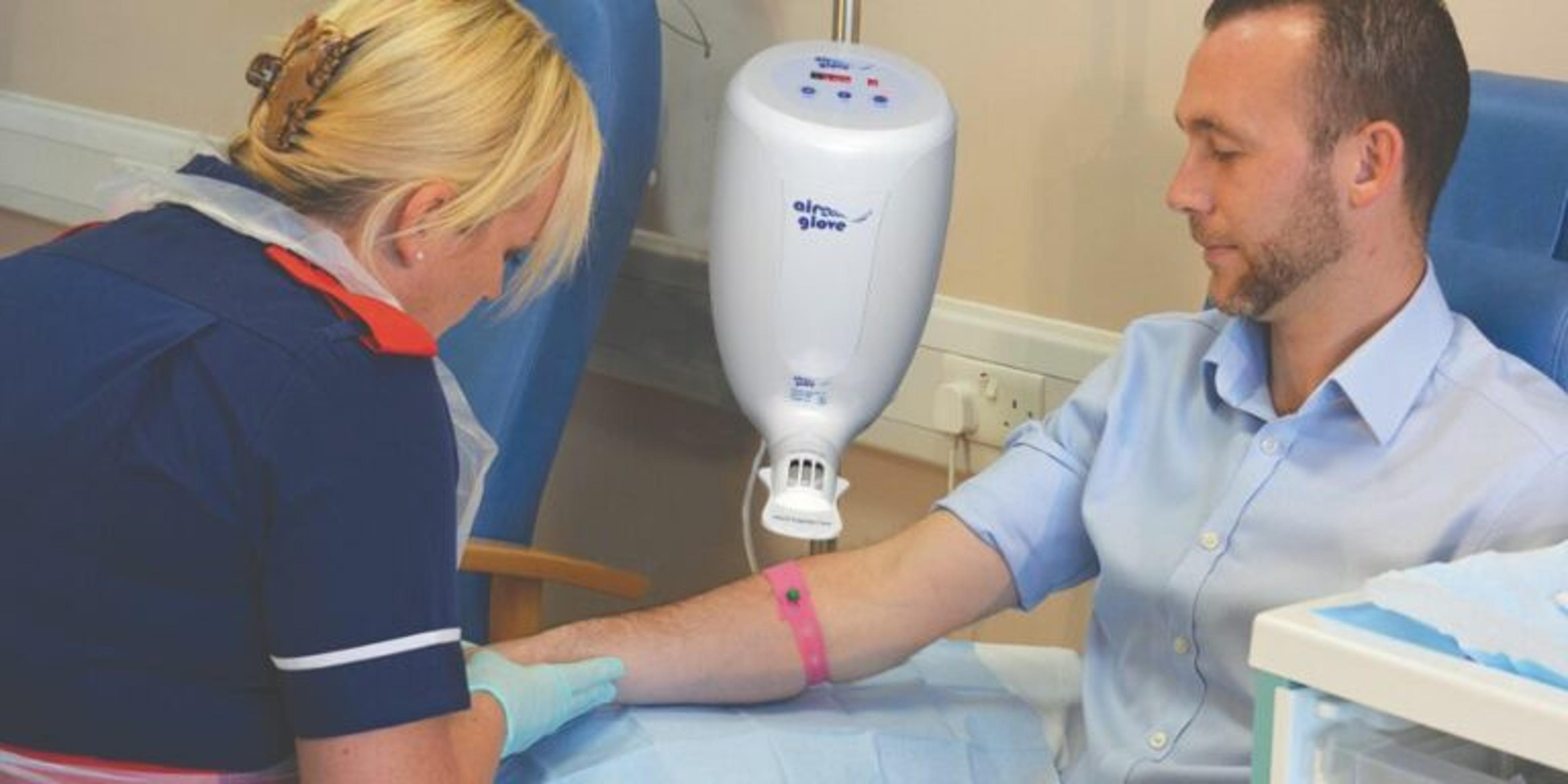
Airglove v2 tackles this problem with a deceptively simple solution: a double‑walled polyethylene glove that circulates warm air over the patient’s arm. The controlled heat raises skin and blood temperature, causing vasodilation and making veins more prominent and pliable. Real‑world data from oncology departments—including the Royal Marsden and Guy’s Hospital—showed an 87.5% first‑time success rate, a stark improvement over the national average. Clinicians report smoother insertions, reduced need for multiple attempts, and higher patient satisfaction, while staff benefit from streamlined workflows and fewer procedural interruptions.
The broader market implications are significant. With a patented, globally protected technology, Airglove positions itself to capture a sizable share of the IV access market, which is projected to grow as healthcare systems prioritize patient‑centred care and cost containment. Early adoption in high‑volume oncology units could catalyze wider deployment across emergency, critical care, and outpatient settings. As hospitals increasingly seek evidence‑based tools to enhance safety and efficiency, Airglove’s blend of low‑tech hardware and high‑impact results may set a new standard for vein preparation, prompting competitors to explore similar physiologic augmentation approaches.
Airglove Medical announces major breakthrough in difficult venous access
Not provided
Scottish company Airglove Medical has announced the launch of Airglove v2, an air‑warming system developed to enhance vein physiology prior to venepuncture and improve access in patients with difficult intravenous access. The company says it is a proven patient solution to make cannulation easier, faster and more comfortable for the benefit of patients and staff.
The award‑winning system was officially launched at the UKONS conference in Birmingham following extensive and highly successful oncology trials in more than 150 UK hospitals since 2018, including Royal Marsden Cancer Centre, Guy’s and St Thomas’ Hospital and St Barts Hospital.
The initial patient‑service evaluation achieved 87.5 % first‑time cannulation in NHS Maidstone & Tunbridge Wells Oncology Department, with oncology being regarded as having some of the most difficult‑to‑access veins.
With over 32 million cannulations per year in the UK alone and a reported 30 % failure rate of accessing the vein on the first attempt, this worldwide‑patented air‑warming system is set to transform the market. It gently heats the patient’s arm as it forces warm air through a double‑walled polyethylene (LDPE) glove, warming the underlying structures and blood, which dilates the veins.
“Our mission at Airglove is to positively impact the lives of patients, in a small but mighty way. Protecting precious veins is vital through a treatment journey, which has a physical and mental impact. We want to balance the scales of the unintended consequences of IV access through vein preparation and reducing anxiety.”
— Jason Ram, Chief Executive Officer, Airglove Medical
Airglove aims to improve workflow and reduce procedure delays, with positive feedback received from both staff and patients. It is an example of how simple innovation can solve complex problems, bridging the gap between technology and care.
0
Comments
Want to join the conversation?
Loading comments...